 Add My Company
Add My Company
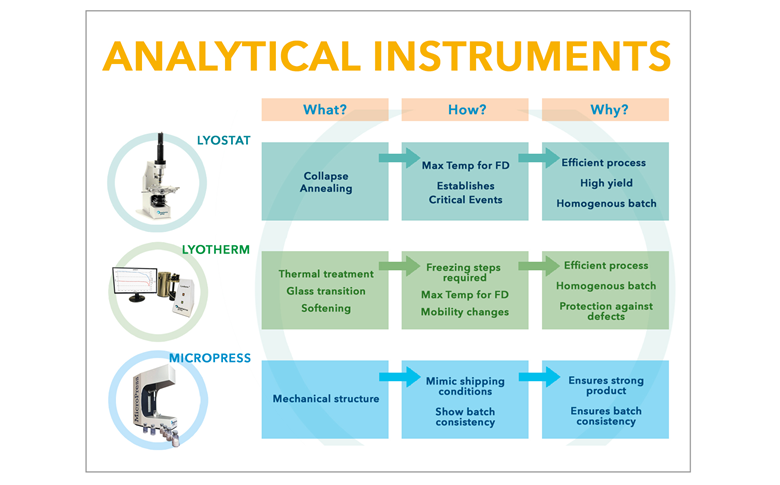
A rational, knowledge-based approach to designing and developing successful freeze drying formulations and cycles requires information about how the product responds to different processing conditions. Candidate formulations should be analysed in terms of their visible physical structure, thermal characteristics and frozen state mobility, in order to gain a sufficient understanding of its behaviour throughout the freeze drying process. This ensures that cycles can be developed that are tailored to the specific needs of every product, making them efficient, safe, robust and reproducible.
With each product and process recognised as unique, by entrusting us with your project, you will benefit from our depth of experience and knowledge to ultimately reach any project goal. Each project will be carefully managed by Biopharma Group?s team of scientists and consultants. Through the operation of proprietary and advanced equipment, both R&D and manufacturing activities will run efficiently.
For further information visit our website, leave us a message or find the contact details to speak to our specialist team.
For more information on Designers Of Analytical instruments International talk to Biopharma Group

