 Add My Company
Add My Company
Sign In
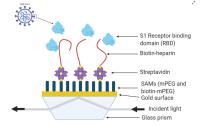
In search of therapeutic treatments that can inhibit the infection of SARS-CoV-2 viruses that cause COVID-19 in humans, heparin, as well as heparan sulphate (HS) and its derivatives, may be potential drug candidates due to their known binding interactions with surface viral proteins [1]. Furthermore, they are inexpensive and widely available. Heparin is a glucosaminoglycan (GAG), which is a complex carbohydrate found on animal cells. It is known that these GAGs bind to surface viral proteins which result in viral adhesion and cell entry, hence allowing infections to take place [1]. In addition, the spatial arrangement of sulphated groups on heparin and its derivatives may influence how effectively viral proteins can bind to them [1].
Surface plasmon resonance (SPR) is a very effective way to determine the presence of binding interactions between proteins and their ligands without the use of any labels in real-time. It is also a very rapid method to evaluate a pair of drug-receptor interactions compared to doing immunoassays such as ELISA, which are, in contrast, very laborious, time-consuming, and require expensive reagents. In the study by Mycroft-West et al., SPR, specifically Affinit??s quad inlet P4SPR?, was used to confirm the binding between SARS-CoV-2 S1 receptor-binding domain (RBD) and heparin [1]. A competitive assay also demonstrated the extent at which each heparin derivative could inhibit the binding between the RBD and immobilized heparin on the SPR sensor chip. This blog provides the details as to how the research team used the P4SPR to find the heparin derivative that most inhibited the binding between RBD and surface-immobilized heparin.
SPR Experimental Details
A quad inlet P4SPR? (4 channels) was used to measure the binding interactions between heparin and RBD. This was done by immobilizing heparin onto SPR sensor chips via a biotin-streptavidin linkage and measuring the SPR shift upon introduction of the RBD. First, the P4SPR gold sensor chips were functionalized with a mixture of polyethylene glycol methyl ether (mPEG) thiol and biotin-mPEG thiol [1]. Streptavidin was injected into the channels followed by biotin-heparin (but not injected into the reference channel) [1]. Ligands such as human fibroblast growth factor 2 (FGF2) and SARS-CoV-2 S1 RBD were then introduced into all the channels in the P4SPR [1]. An average SPR signal was obtained from the 3 measurement channels.
Confirmation of Binding with FGF2 and SARS-CoV-2 S1 RBD Protein using P4SPR?
FBF2 was first introduced to the heparin-modified SPR sensor chips to validate the SPR assay as it is known to bind to heparin or HS [2]. Injection of 100 nM FGF2 led to a SPR shift of 1.60 nm (or 567 RU) [1]. The control channel, which had no heparin, did not elicit a signal. In a separate experiment, the injection of 800 nM SARS-CoV-2 S1 RBD led to a signal shift of 0.22 nm (or 78 RU) [1]. The control channel only showed at most 10% of the signal observed in the channels containing heparin [1]. Therefore, these two initial assays demonstrated that heparin bound to both FGF2 and SARS-CoV-2 S1 RBD proteins. Furthermore, these signals could be observed in real-time and measured within 3 min.
An SPR Competition Assay with Heparin and other Heparin Derivatives
Mixtures of 800 nM SARS-CoV-2 S1 RBD and heparin of various concentrations were prepared and injected into the P4SPR. Once the SPR response increased and started to stabilize, PBST buffer was injected again and the SPR shift was measured. Starting at 1.7 ug/mL of heparin, a reduction in the binding of SARS-CoV-2 S1 RBD to the heparin was observed [1]. The signal further decreased and followed a dose-dependent manner, and the binding was completely inhibited at 1.7 mg/mL of heparin [1]. As a comparison, a low-molecular weight heparin called enoxaparin, was also mixed with SARS-CoV-2 S1 RBD prior to being injected over the heparin-immobilized surfaces. It was found to be not as potent as heparin since it was only 70% effective at inhibiting SARS-CoV-2 S1 RBD from binding to the surface-immobilized heparin at 1.7 mg/mL of enoxaparin [1].
In a separate SPR experiment, a panel of heparin derivatives of different levels and patterns of sulfation was used to compete with surface-immobilized heparin to bind to SARS-CoV-2 S1 RBD. The rationale for this experiment is that the extent of sulfation influences the conformation of the GAGs, which would affect its binding to the RBD [1]. The result of this part of the study showed that some derivatives such as enoxaparin and Hep9 prevented more than 50% of RBD from binding to immobilized heparin while others (e.g. Hep8) did not have such a significant effect (see Fig. 2) [1]. Still, native heparin inhibited binding of RBD to surface-immobilized heparin most effectively by only allowing less than 10% of RBD to bind to immobilized heparin [1]. This demonstrates that the degree and location of sulfation dictate the binding between SARS-CoV-2 S1 RBD and these heparin derivatives. Most importantly, the P4SPR was able to quantify the degree of inhibition by these heparin derivatives.
Conclusions
By immobilizing heparin onto SPR sensor chips via a biotin-streptavidin linkage, the binding between the SARS-CoV-2 S1 RBD and heparin was quickly validated. Furthermore, the extent of binding inhibition was measured for heparin, HS and its derivatives using a user-friendly SPR setup. The quad inlet P4SPR provides a simple yet informative tool that can pave the way to discover therapeutic drugs to prevent infection by the SARS-CoV-2 and other viruses.
The Affinit? Advantage
Affinit? Instruments? P4SPR? is a very user-friendly, compact, and portable instrument. In addition, samples do not need much preparation and can be manually injected into the instrument. The P4SPR?, compared to a traditional immunoassay such as ELISA, provides fast, real-time affinity and/or kinetic data.
Simplicity ? Fast training, fast results
Versatility ? Pharmaceutical, biosensing, assay development applications
Economy ? Affordable, accessible
We help life science labs and biotech companies to do rapid assay development and characterization. Feel free to reach out to us about the expertise we offer at info@meritics.com
References
[1] Courtney J. Mycroft-West, Dunhao Su, Isabel Pagani, Timothy R. Rudd, Stefano Elli, Neha S. Gandhi, Scott E. Guimond, Gavin J. Miller, Maria C. Z. Meneghetti, Helena B. Nader, Yong Li, Quentin M. Nunes, Patricia Procter, Nicasio Mancini, Massimo Clementi, Antonella Bisi, Nicholas R. Forsyth, Vito Ferro, Jeremy E. Turnbull, Marco Guerrini, David G. Fernig, Elisa Vicenzi, Edwin A. Yates, Marcelo A. Lima, Mark A. Skidmore. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost., 2020,120, 1700.
[2] Zusana Koledova, Jakub Sumbal, Anas Rabata, Gabin de la Bourdonnaye, Radha Chaloupkova, Barbara Hrdlickova, Jiri Damborsky, Veronika Stepankova. Fibroblast Growth Factor 2 Protein Stability Provides Decreased Dependence on Heparin for Induction of FGFR Signaling and Alters ERK Signaling Dynamics. Front. Cell Dev. Biol., 2019, 7, Article 331.
For more information on Binding Interaction Studies Between Heparin and SARS-CoV-2 by Using P4SPR? talk to Meritics Ltd
Enquire Now
List your company on FindTheNeedle.

