 Add My Company
Add My Company
Sign In
Antibodies-based therapeutics are perhaps the most successful and versatile drug approach developed in the last 30 years. They not only have found application as vaccines and drug carriers but have also been applied to analytical methods such as ELISA (Enzyme-linked Immunosorbent Assay), Wester blotting and flow cytometry.
The discovery of the smallpox vaccine by Jenner in the late 1700s certainly represents the paradigm shift for the most disparate applications of antibodies currently available in the biomedical market.
Antibodies have evolved considerably from early stages and now are developed as the new generation – called Biobetter – which aim to minimise some of the typical drawbacks such as limited efficacy due to poor tissue and tumour penetration, low in vivo efficacy, cumbersome administration, limited solubility, aggregation and high production costs.
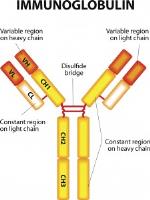
In response, immunoglobulin-miniobetters are formulated and administrated in many forms and portions (Figure 1-2), where their constituents, i.e. fragments, have shown to be more suitable than their intact equivalent. This is the case of the engineered antibody Fab – Fragment antigen-binding – where smaller sizes are critically important to access sterically hindered antigens. Solid-tumour penetration, for example, has proven to be one of the most effective targets. The main drawback of antibody fragment-based approaches, however, lies in there being a faster clearance from circulation which leads to a shorter half-life in vivo.
Antibodies can also be conjugated with other molecules making ADC –antibody drug-conjugates. Conjugates are typically immunoglobulins or other chemical entities such as oligonucleotides and peptides (Figure 2). These are typically cytotoxic molecules with Mw of 300–1,000 Da – called warheads – bound via synthetic linkers. Such immunoconjugates combine the high selectivity, stability and favourable pharmacokinetic profile of mAbs, with the antitumour potency of highly cytotoxic small-molecule drugs, with an inhibitory concentration in the range of subnanomolar concentrations.
The production of antibodies occurs mainly in mammalian host cell lines because of their ability to introduce post-translational modifications. The most widely used cell line is Chinese hamster ovary (CHO), followed by mouse cell lines SP2/0 and NS0 and hybridomas. Bacterial strains such as Escherichia coli have also proven to be a good alternative in Fabs production. The advantages of microbial cells rely on fast growth, well-characterised genetics and low cultivation costs.
Besides from biological, chemical approaches are also widely used when a parental monoclonal antibody is cut and reconstituted in order to produce a bifunctional antibody. This approach makes monospecific antibodies, bifunctional and bispecific (BsAb). In addition, the absence of glycosylated sites, allows BsAb to be easily produced in bacterial cells.
Figure 2: (ADC) Antibody-Drug Conjugate Components
The extraordinary success of antibody-based therapies in new emerging markets and patent expiration of biologic products has opened a wide range of opportunities for players in the biosimilars market. By definition, a biosimilar represents a copy of an approved biological product, also known as the reference product, and shows no differences in terms of safety and effectiveness. This offers a great opportunity for any potential developer to produce equivalents – but not exact copies – of the reference product. In other words, it represents a broader field of development which differs from the general concept of generic drugs, which are the exact copy of the blockbuster, differing in the excipients only.
From a commercial perspective, the global biosimilars market reached $1.8 billion in 2013 and nearly $2.0 billion in 2014. By 2016 it rose to $3.39 billion and is estimated to reach $10.90 billion by 2021 (Figure 3). In the next three years, the combined worldwide sales for all antibodies-based drug therapies will be nearly $125 billion combined, but the industry reveals the down-side is the challenges they face in development complexities related to R&D, manufacturing and marketing.
At present, there are about 30 companies focussing on antibody development but only in the last 2 years they have seen approved 14 distinct molecules. Moreover, the pipeline includes 20 cases that have either completed the trials (6) or are currently in Phase III (14), with an additional 8 major products expected to lose exclusivity by 2020.
Since the first therapeutic monoclonal antibody – Orthoclone OKT3 – was approved for the prevention of kidney transplant rejection in 1986, antibody-related products have taken a long journey becoming the principal target in the current pharmaceutical market. It is accepted, however, that many of the critical points are still concerns of the investigation, especially when related to the reduction of high costs, poor efficacy, stability and most importantly, guaranteeing a greater patient benefit.
For more information on ADCs and BIOSIMILARS: The development towards a new generation of targeted therapies talk to Biopharma Group
Enquire Now
More News
List your company on FindTheNeedle.

